From Wikipedia, the free encyclopedia
Quantum biology is the study of applications of quantum mechanics and theoretical chemistry to aspects of biology that cannot be accurately described by the classical laws of physics.[1] An understanding of fundamental quantum interactions is important because they determine the properties of the next level of organization in biological systems.
Many biological processes involve the conversion of energy into forms that are usable for chemical transformations, and are quantum mechanical in nature. Such processes involve chemical reactions, light absorption, formation of excited electronic states, transfer of excitation energy, and the transfer of electrons and protons (hydrogen ions) in chemical processes, such as photosynthesis, olfaction and cellular respiration.[2] Moreover, quantum biology may use computations to model biological interactions in light of quantum mechanical effects.[3] Quantum biology is concerned with the influence of non-trivial quantum phenomena,[4] which can be explained by reducing the biological process to fundamental physics, although these effects are difficult to study and can be speculative.[5]
Currently, there exist four major life processes that have been identified as influenced by quantum effects:
- enzyme catalysis,
- sensory processes,
- energy transference, and
- information encoding.[6]
History[edit]
Quantum biology is an emerging field, in the sense that most current research is theoretical and subject to questions that require further experimentation. Though the field has only recently received an influx of attention, it has been conceptualized by physicists throughout the 20th century. It has been suggested that quantum biology might play a critical role in the future of the medical world.[7] Early pioneers of quantum physics saw applications of quantum mechanics in biological problems. Erwin Schrödinger's 1944 book What Is Life? discussed applications of quantum mechanics in biology.[8]
- Schrödinger introduced the idea of an "aperiodic crystal" that contained genetic information in its configuration of covalent chemical bonds.
- He further suggested that mutations are introduced by "quantum leaps".
Other pioneers Niels Bohr, Pascual Jordan, and Max Delbrück argued that the quantum idea of complementarity was fundamental to the life sciences.[9] In 1963, Per-Olov Löwdin published proton tunneling as another mechanism for DNA mutation. In his paper, he stated that there is a new field of study called "quantum biology".[10] In 1979, the Soviet and Ukrainian physicist Alexander Davydov published the first textbook on quantum biology entitled Biology and Quantum Mechanics.[11][12]
Enzyme catalysis[edit]
Enzymes have been postulated to use quantum tunneling to transfer electrons in electron transport chains.[13][14][15] It is possible that protein quaternary architectures may have adapted to enable sustained quantum entanglement and coherence, which are two of the limiting factors for quantum tunneling in biological entities.[16] These architectures might account for a greater percentage of quantum energy transfer, which occurs through electron transport and proton tunneling (usually in the form of hydrogen ions, H+).[17][18] Tunneling refers to the ability of a subatomic particle to travel through potential energy barriers.[19] This ability is due, in part, to the principle of complementarity, which holds that certain substances have pairs of properties that cannot be measured separately without changing the outcome of measurement. Particles, such as electrons and protons, have wave-particle duality; they can pass through energy barriers due to their wave characteristics without violating the laws of physics. In order to quantify how quantum tunneling is used in many enzymatic activities, many biophysicists utilize the observation of hydrogen ions. When hydrogen ions are transferred, this is seen as a staple in an organelle's primary energy processing network; in other words, quantum effects are most usually at work in proton distribution sites at distances on the order of an angstrom (1 Å).[20][21] In physics, a semiclassical (SC) approach is most useful in defining this process because of the transfer from quantum elements (e.g. particles) to macroscopic phenomena (e.g. biochemicals). Aside from hydrogen tunneling, studies also show that electron transfer between redox centers through quantum tunneling plays an important role in enzymatic activity of photosynthesis and cellular respiration (see also Mitochondria section below).[15][22]
Ferritin[edit]
Ferritin is an iron storage protein that is found in plants and animals. It is usually formed from 24 subunits that self-assemble into a spherical shell that is approximately 2 nm thick, with an outer diameter that varies with iron loading up to about 16 nm. Up to ~4500 iron atoms can be stored inside the core of the shell in the Fe3+ oxidation state as water-insoluble compounds such as ferrihydrite and magnetite.[23] Ferritin is able to store electrons for at least several hours, which reduce the Fe3+ to water soluble Fe2+.[24] Electron tunneling as the mechanism by which electrons transit the 2 nm thick protein shell was proposed as early as 1988.[25] Electron tunneling and other quantum mechanical properties of ferritin were observed in 1992,[26] and electron tunneling at room temperature and ambient conditions was observed in 2005.[27] Electron tunneling associated with ferritin is a quantum biological process, and ferritin is a quantum biological agent.
Electron tunneling through ferritin between electrodes is independent of temperature, which indicates that it is substantially coherent and activation-less.[28] The electron tunneling distance is a function of the size of the ferritin. Single electron tunneling events can occur over distances of up to 8 nm through the ferritin, and sequential electron tunneling can occur up to 12 nm through the ferritin. It has been proposed that the electron tunneling is magnon-assisted and associated with magnetite microdomains in the ferritin core.[29]
Early evidence of quantum mechanical properties exhibited by ferritin in vivo was reported in 2004, where increased magnetic ordering of ferritin structures in placental macrophages was observed using small angle neutron scattering (SANS).[30] Quantum dot solids also show increased magnetic ordering in SANS testing,[31] and can conduct electrons over long distances.[32] Increased magnetic ordering of ferritin cores disposed in an ordered layer on a silicon substrate with SANS testing has also been observed.[33] Ferritin structures like those in placental macrophages have been tested in solid state configurations and exhibit quantum dot solid-like properties of conducting electrons over distances of up to 80 microns through sequential tunneling and formation of Coulomb blockades.[34][35][36] Electron transport through ferritin in placental macrophages may be associated with an anti-inflammatory function.[37]
Conductive atomic force microscopy of substantia nigra pars compacta (SNc) tissue demonstrated evidence of electron tunneling between ferritin cores, in structures that correlate to layers of ferritin outside of neuromelanin organelles.[38]
Evidence of ferritin layers in cell bodies of large dopamine neurons of the SNc and between those cell bodies in glial cells has also been found,[39][40][41] and is hypothesized to be associated with neuron function.[42] Overexpression of ferritin reduces the accumulation of reactive oxygen species (ROS),[43] and may act as a catalyst by increasing the ability of electrons from antioxidants to neutralize ROS through electron tunneling. Ferritin has also been observed in ordered configurations in lysosomes associated with erythropoiesis,[44] where it may be associated with red blood cell production. While direct evidence of tunneling associated with ferritin in vivo in live cells has not yet been obtained, it may be possible to do so using QDs tagged with anti-ferritin, which should emit photons if electrons stored in the ferritin core tunnel to the QD.[45]

Electron microscope image of placental macrophage ferritin

Conductive atomic force microscopy image of human substantia nigra pars compacta (SNc) tissue

Electron spectroscopic imaging of iron (red) outside of neuromelanin organelles
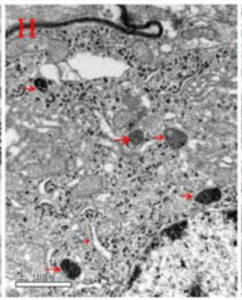
Electron microscope image of glial cell from SNc showing structures similar to ferritin in placental tissue
Sensory processes[edit]
Olfaction[edit]
Olfaction, the sense of smell, can be broken down into two parts; the reception and detection of a chemical, and how that detection is sent to and processed by the brain. This process of detecting an odorant is still under question. One theory named the “shape theory of olfaction” suggests that certain olfactory receptors are triggered by certain shapes of chemicals and those receptors send a specific message to the brain.[46] Another theory (based on quantum phenomena) suggests that the olfactory receptors detect the vibration of the molecules that reach them and the “smell” is due to different vibrational frequencies, this theory is aptly called the “vibration theory of olfaction.”
The vibration theory of olfaction, created in 1938 by Malcolm Dyson[47] but reinvigorated by Luca Turin in 1996,[48] proposes that the mechanism for the sense of smell is due to G-protein receptors that detect molecular vibrations due to inelastic electron tunneling, tunneling where the electron loses energy, across molecules.[48] In this process a molecule would fill a binding site with a G-protein receptor. After the binding of the chemical to the receptor, the chemical would then act as a bridge allowing for the electron to be transferred through the protein. As the electron transfers across what would otherwise have been a barrier, it loses energy due to the vibration of the newly-bound molecule to the receptor. This results in the ability to smell the molecule.[48][4]
While the vibration theory has some experimental proof of concept,[49][50] there have been multiple controversial results in experiments. In some experiments, animals are able to distinguish smells between molecules of different frequencies and same structure,[51] while other experiments show that people are unaware of distinguishing smells due to distinct molecular frequencies.[52]
Vision[edit]
Main article: Visual phototransduction
Vision relies on quantized energy in order to convert light signals to an action potential in a process called phototransduction. In phototransduction, a photon interacts with a chromophore in a light receptor. The chromophore absorbs the photon and undergoes photoisomerization. This change in structure induces a change in the structure of the photo receptor and resulting signal transduction pathways lead to a visual signal. However, the photoisomerization reaction occurs at a rapid rate, in under 200 femtoseconds,[53] with high yield. Models suggest the use of quantum effects in shaping the ground state and excited state potentials in order to achieve this efficiency.[54]
The sensor in the retina of the human eye is sensitive enough to detect a single photon.[55] Single photon detection could lead to multiple different technologies. One area of development is in quantum communication and cryptography. The idea is to use a biometric system to measure the eye using only a small number of points across the retina with random flashes of photons that “read” the retina and identify the individual.[56] This biometric system would only allow a certain individual with a specific retinal map to decode the message. This message can not be decoded by anyone else unless the eavesdropper were to guess the proper map or could read the retina of the intended recipient of the message.[57]
Energy transfer[edit]
Photosynthesis[edit]
Main article: Photosynthesis
 Generic photosystem Complex
Generic photosystem Complex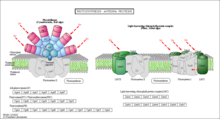 Antennae complex found in photosystems of both prokaryotes and eukaryotes
Antennae complex found in photosystems of both prokaryotes and eukaryotes Diagram of FMO complex. Light excites electrons in an antenna. The excitation then transfers through various proteins in the FMO complex to the reaction center to further photosynthesis.
Diagram of FMO complex. Light excites electrons in an antenna. The excitation then transfers through various proteins in the FMO complex to the reaction center to further photosynthesis.Photosynthesis refers to the biological process that photosynthetic cells use to synthesize organic compounds from inorganic starting materials using sunlight.[58] What has been primarily implicated as exhibiting non-trivial quantum behaviors is the light reaction stage of photosynthesis. In this stage, photons are absorbed by the membrane-bound photosystems. Photosystems contain two major domains, the light-harvesting complex (antennae) and the reaction center. These antennae vary among organisms. For example, bacteria use circular aggregates of chlorophyll pigments, while plants use membrane-embedded protein and chlorophyll complexes.[59][60] Regardless, photons are first captured by the antennae and passed on to the reaction-center complex. Various pigment-protein complexes, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to reaction site. The photon-driven excitation of the reaction-center complex mediates the oxidation and the reduction of the primary electron acceptor, a component of the reaction-center complex. Much like the electron transport chain of the mitochondria, a linear series of oxidations and reductions drives proton (H+) pumping across the thylakoid membrane, the development of a proton motive force, and energetic coupling to the synthesis of ATP.
Previous understandings of electron-excitation transference (EET) from light-harvesting antennae to the reaction center have relied on the Förster theory of incoherent EET, postulating weak electron coupling between chromophores and incoherent hopping from one to another. This theory has largely been disproven by FT electron spectroscopy experiments that show electron absorption and transfer with an efficiency of above 99%,[61] which cannot be explained by classical mechanical models. Instead, as early as 1938, scientists theorized that quantum coherence was the mechanism for excitation-energy transfer. Indeed, the structure and nature of the photosystem places it in the quantum realm, with EET ranging from the femto- to nanosecond scale, covering sub-nanometer to nanometer distances.[62] The effects of quantum coherence on EET in photosynthesis are best understood through state and process coherence. State coherence refers to the extent of individual superpositions of ground and excited states for quantum entities, such as excitons. Process coherence, on the other hand, refers to the degree of coupling between multiple quantum entities and their evolution as either dominated by unitary or dissipative parts, which compete with one another. Both of these types of coherence are implicated in photosynthetic EET, where a exciton is coherently delocalized over several chromophores.[63] This delocalization allows for the system to simultaneously explore several energy paths and use constructive and destructive interference to guide the path of the exciton's wave packet. It is presumed that natural selection has favored the most efficient path to the reaction center. Experimentally, the interaction between the different frequency wave packets, made possible by long-lived coherence, will produce quantum beats.[64]
While quantum photosynthesis is still an emerging field, there have been many experimental results that support the quantum-coherence understanding of photosynthetic EET. A 2007 study claimed the identification of electronic quantum coherence[65] at −196 °C (77 K). Another theoretical study from 2010[which?] provided evidence that quantum coherence lives as long as 300 femtoseconds at biologically relevant temperatures (4 °C or 277 K). In that same year, experiments conducted on photosynthetic cryptophyte algae using two-dimensional photon echo spectroscopy yielded further confirmation for long-term quantum coherence.[66] These studies suggest that, through evolution, nature has developed a way of protecting quantum coherence to enhance the efficiency of photosynthesis. However, critical follow-up studies question the interpretation of these results. Single-molecule spectroscopy now shows the quantum characteristics of photosynthesis without the interference of static disorder, and some studies use this method to assign reported signatures of electronic quantum coherence to nuclear dynamics occurring in chromophores.[67][68][69][70][71][72][73] A number of proposals emerged to explain unexpectedly long coherence. According to one proposal, if each site within the complex feels its own environmental noise, the electron will not remain in any local minimum due to both quantum coherence and its thermal environment, but proceed to the reaction site via quantum walks.[74][75][76] Another proposal is that the rate of quantum coherence and electron tunneling create an energy sink that moves the electron to the reaction site quickly.[77] Other work suggested that geometric symmetries in the complex may favor efficient energy transfer to the reaction center, mirroring perfect state transfer in quantum networks.[78] Furthermore, experiments with artificial dye molecules cast doubts on the interpretation that quantum effects last any longer than one hundred femtoseconds.[79]
In 2017, the first control experiment with the original FMO protein under ambient conditions confirmed that electronic quantum effects are washed out within 60 femtoseconds, while the overall exciton transfer takes a time on the order of a few picoseconds.[80] In 2020 a review based on a wide collection of control experiments and theory concluded that the proposed quantum effects as long lived electronic coherences in the FMO system does not hold.[81] Instead, research investigating transport dynamics suggests that interactions between electronic and vibrational modes of excitation in FMO complexes require a semi-classical, semi-quantum explanation for the transfer of exciton energy. In other words, while quantum coherence dominates in the short-term, a classical description is most accurate to describe long-term behavior of the excitons.[82][83]
Another process in photosynthesis that has almost 100% efficiency is charge transfer, again suggesting that quantum mechanical phenomena are at play.[73] In 1966, a study on the photosynthetic bacterium Chromatium found that at temperatures below 100 K, cytochrome oxidation is temperature-independent, slow (on the order of milliseconds), and very low in activation energy. The authors, Don DeVault and Britton Chase, postulated that these characteristics of electron transfer are indicative of quantum tunneling, whereby electrons penetrate a potential barrier despite possessing less energy than is classically necessary.
Mitochondria[edit]
Mitochondria have been demonstrated to utilize quantum tunneling in their function as the powerhouse of eukaryotic cells. Similar to the light reactions in the thylakoid, linearly-associated membrane-bound proteins comprising the electron transport chain (ETC) energetically link the reduction of O2 with the development of a proton motive gradient (H+) across the inner membrane of the mitochondria. This energy stored as a proton motive gradient is then coupled with the synthesis of ATP. It is significant that the mitochondrion conversion of biomass into chemical ATP achieves 60-70% thermodynamic efficiency, far superior to that of man-made engines.[84] This high degree of efficiency is largely attributed to the quantum tunnelling of electrons in the ETC and of protons in the proton motive gradient. Indeed, electron tunneling has already been demonstrated in certain elements of the ETC including NADH:ubiquinone oxidoreductase(Complex I) and CoQH2-cytochrome c reductase (Complex III).[85][86]
In quantum mechanics, both electrons and protons are quantum entities that exhibit wave-particle duality, exhibiting both particle and wave-like properties depending on the method of experimental observation.[87] Quantum tunneling is a direct consequence of this wave-like nature of quantum entities that permits the passing-through of a potential energy barrier that would otherwise restrict the entity.[88] Moreover, it depends on the shape and size of a potential barrier relative to the incoming energy of a particle.[89] Because the incoming particle is defined by its wave function, its tunneling probability is dependent upon the potential barrier's shape in an exponential way. For example, if the barrier is relatively wide, the incoming particle's probability to tunnel will decrease. The potential barrier, in some sense, can come in the form of an actual biomaterial barrier. The inner mitochondria membrane which houses the various components of the ETC is on the order of 7.5 nm thick.[84] The inner membrane of a mitochondrion must be overcome to permit signals (in the form of electrons, protons, H+) to transfer from the site of emittance (internal to the mitochondria) and the site of acceptance (i.e. the electron transport chain proteins).[90] In order to transfer particles, the membrane of the mitochondria must have the correct density of phospholipids to conduct a relevant charge distribution that attracts the particle in question. For instance, for a greater density of phospholipids, the membrane contributes to a greater conductance of protons.[90]
Molecular solitons in proteins[edit]
Main article: Davydov soliton
Alexander Davydov developed the quantum theory of molecular solitons in order to explain the transport of energy in protein α-helices in general and the physiology of muscle contraction in particular.[91][92] He showed that the molecular solitons are able to preserve their shape through nonlinear interaction of amide I excitons and phonon deformations inside the lattice of hydrogen-bonded peptide groups.[93][94] In 1979, Davydov published his complete textbook on quantum biology entitled "Biology and Quantum Mechanics" featuring quantum dynamics of proteins, cell membranes, bioenergetics, muscle contraction, and electron transport in biomolecules.[11][12]
Information encoding[edit]
Magnetoreception[edit]
Main article: Magnetoreception
 The radical pair mechanism has been proposed for quantum magnetoreception in birds. It takes place in cryptochrome molecules in cells in the birds' retinas.[95]
The radical pair mechanism has been proposed for quantum magnetoreception in birds. It takes place in cryptochrome molecules in cells in the birds' retinas.[95]Magnetoreception is the ability of animals to navigate using the inclination of the magnetic field of the Earth.[96] A possible explanation for magnetoreception is the entangled radical pair mechanism.[97][98] The radical-pair mechanism is well-established in spin chemistry,[99][100][101] and was speculated to apply to magnetoreception in 1978 by Schulten et al.. The ratio between singlet and triplet pairs is changed by the interaction of entangled electron pairs with the magnetic field of the Earth.[102] In 2000, cryptochrome was proposed as the "magnetic molecule" that could harbor magnetically sensitive radical-pairs. Cryptochrome, a flavoprotein found in the eyes of European robins and other animal species, is the only protein known to form photoinduced radical-pairs in animals.[96] When it interacts with light particles, cryptochrome goes through a redox reaction, which yields radical pairs both during the photo-reduction and the oxidation. The function of cryptochrome is diverse across species, however, the photoinduction of radical-pairs occurs by exposure to blue light, which excites an electron in a chromophore.[102] Magnetoreception is also possible in the dark, so the mechanism must rely more on the radical pairs generated during light-independent oxidation.
Experiments in the lab support the basic theory that radical-pair electrons can be significantly influenced by very weak magnetic fields, i.e., merely the direction of weak magnetic fields can affect radical-pair's reactivity and therefore can "catalyze" the formation of chemical products. Whether this mechanism applies to magnetoreception and/or quantum biology, that is, whether Earth's magnetic field "catalyzes" the formation of biochemical products by the aid of radical-pairs, is not fully clear. Radical-pairs may need not be entangled, the key quantum feature of the radical-pair mechanism, to play a part in these processes. There are entangled and non-entangled radical-pairs, but disturbing only entangled radical-pairs is not possible with current technology. Researchers found evidence for the radical-pair mechanism of magnetoreception when European robins, cockroaches, and garden warblers, could no longer navigate when exposed to a radio frequency that obstructs magnetic fields[96] and radical-pair chemistry. Further evidence came from a comparison of Cryptochrome 4 (CRY4) from migrating and non-migrating birds. CRY4 from chicken and pigeon were found to be less sensitive to magnetic fields than those from the (migrating) European robin, suggesting evolutionary optimization of this protein as a sensor of magnetic fields.[103]
DNA mutation[edit]
DNA acts as the instructions for making proteins throughout the body. It consists of 4 nucleotides: guanine, thymine, cytosine, and adenine.[104] The order of these nucleotides gives the "recipe" for the different proteins.
Whenever a cell reproduces, it must copy these strands of DNA. However, sometime throughout the process of copying the strand of DNA a mutation, or an error in the DNA code, can occur. A theory for the reasoning behind DNA mutation is explained in the Lowdin DNA mutation model.[105] In this model, a nucleotide may spontaneously change its form through a process of quantum tunneling.[106][107] Because of this, the changed nucleotide will lose its ability to pair with its original base pair and consequently change the structure and order of the DNA strand.
Exposure to ultraviolet light and other types of radiation can cause DNA mutation and damage. The radiation also can modify the bonds along the DNA strand in the pyrimidines and cause them to bond with themselves, creating a dimer.[108]
In many prokaryotes and plants, these bonds are repaired by a DNA-repair-enzyme photolyase. As its prefix implies, photolyase is reliant on light in order to repair the strand. Photolyase works with its cofactor FADH, flavin adenine dinucleotide, while repairing the DNA. Photolyase is excited by visible light and transfers an electron to the cofactor FADH. FADH - now in the possession of an extra electron - transfers the electron to the dimer to break the bond and repair the DNA. The electron tunnels from the FADH to the dimer. Although the range of this tunneling is much larger than feasible in a vacuum, the tunneling in this scenario is said to be “superexchange-mediated tunneling,” and is possible due to the protein's ability to boost the tunneling rates of the electron.[105]
Other[edit]
Other quantum phenomena in biological systems include the conversion of chemical energy into motion[109] and brownian motors in many cellular processes.[110]
Pseudoscience[edit]
Alongside the multiple strands of scientific inquiry into quantum mechanics has come unconnected pseudoscientific interest; this caused scientists to approach quantum biology cautiously.[111]
Hypotheses such as orchestrated objective reduction which postulate a link between quantum mechanics and consciousness have drawn criticism from the scientific community with some claiming it to be pseudoscientific and "an excuse for quackery".[112]
References[edit]
- ^ Kristiansen, Anita. "The future of quantum biology | Royal Society". royalsociety.org. Retrieved 2022-07-11.
- ^ Quantum Biology. University of Illinois at Urbana-Champaign, Theoretical and Computational Biophysics Group.
- ^ Quantum Biology: Powerful Computer Models Reveal Key Biological Mechanism Science Daily Retrieved Oct 14, 2007
- ^ Jump up to:a b Brookes, J. C. (May 2017). "Quantum effects in biology: golden rule in enzymes, olfaction, photosynthesis and magnetodetection". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 473 (2201): 20160822. Bibcode:2017RSPSA.47360822B. doi:10.1098/rspa.2016.0822. PMC 5454345. PMID 28588400.
- ^ Al-Khalili, Jim (24 August 2015), How quantum biology might explain life's biggest questions, retrieved 2018-12-07
- ^ Brookes, Jennifer C. (May 2017). "Quantum effects in biology: golden rule in enzymes, olfaction, photosynthesis and magnetodetection". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 473 (2201): 20160822. Bibcode:2017RSPSA.47360822B. doi:10.1098/rspa.2016.0822. ISSN 1364-5021. PMC 5454345. PMID 28588400.
- ^ Goh, Bey Hing; Tong, Eng Siang; Pusparajah, Priyia (2020). "Quantum Biology: Does quantum physics hold the key to revolutionizing medicine?". Progress in Drug Discovery & Biomedical Science. 3. doi:10.36877/pddbs.a0000130.
- ^ Margulis, Lynn; Sagan, Dorion (1995). What Is Life?. Berkeley: University of California Press. p. 1. ISBN 978-0684810874.
- ^ Joaquim, Leyla; Freira, Olival; El-Hani, Charbel (September 2015). "Quantum Explorers: Bohr, Jordan, and Delbruck Venturing into Biology". Physics in Perspective. 17 (3): 236–250. Bibcode:2015PhP....17..236J. doi:10.1007/s00016-015-0167-7. S2CID 117722573.
- ^ Löwdin, Per-Olov (1966). "Quantum Genetics and the Aperiodic Solid: Some Aspects on the Biological Problems of Heredity, Mutations, Aging, and Tumors in View of the Quantum Theory of the DNA Molecule". Advances in Quantum Chemistry. 2: 213–360. doi:10.1016/S0065-3276(08)60076-3. S2CID 94362884.
- ^ Jump up to:a b Davydov, Alexander S. (1979). Биология и Квантовая Механика [Biology and Quantum Mechanics] (in Russian). Kyiv: Naukova Dumka. OCLC 736440.
- ^ Jump up to:a b Davydov, Alexander S. (1982). Biology and Quantum Mechanics. Oxford: Pergamon Press. ISBN 9780080263922. OCLC 7875407.
- ^ Marcus, R. A. (May 1956). "On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I". The Journal of Chemical Physics. 24 (5): 966–978. Bibcode:1956JChPh..24..966M. doi:10.1063/1.1742723. ISSN 0021-9606. S2CID 16579694.
- ^ "Editorial". Photosynthesis Research. 22 (1): 1. January 1989. Bibcode:1989PhoRe..22....1.. doi:10.1007/BF00114760. PMID 24424672. S2CID 264017354.
- ^ Jump up to:a b Gray, H. B.; Winkler, J. R. (August 2003). "Electron tunneling through proteins". Quarterly Reviews of Biophysics. 36 (3): 341–372. doi:10.1017/S0033583503003913. PMID 15029828. S2CID 28174890.
- ^ Apte, S.P. Quantum biology: Harnessing nano-technology’s last frontier with modified excipients and food ingredients, J. Excipients and Food Chemicals, 5(4), 177–183, 2014
- ^ Glickman, Michael H.; Wiseman, Jeffrey S.; Klinman, Judith P. (January 1994). "Extremely Large Isotope Effects in the Soybean Lipoxygenase-Linoleic Acid Reaction". Journal of the American Chemical Society. 116 (2): 793–794. doi:10.1021/ja00081a060. ISSN 0002-7863.
- ^ Nagel, Z. D.; Klinman, J. P. (August 2006). "Tunneling and dynamics in enzymatic hydride transfer". Chemical Reviews. 106 (8): 3095–3118. doi:10.1002/chin.200643274. PMID 16895320.
- ^ Griffiths, David J. (2005). Introduction to quantum mechanics (2nd ed.). Upper Saddle River, New Jersey: Pearson Prentice Hall. ISBN 0-13-111892-7. OCLC 53926857.
- ^ Masgrau, Laura; Roujeinikova, Anna; Johannissen, Linus O.; et al. (April 2006). "Atomic description of an enzyme reaction dominated by proton tunneling". Science. 312 (5771): 237–241. Bibcode:2006Sci...312..237M. doi:10.1126/science.1126002. PMID 16614214. S2CID 27201250.
- ^ Zewail, Ahmed H. (2008). Physical biology : from atoms to medicine. London, UK: Imperial College Press. ISBN 978-1-84816-201-3. OCLC 294759396.
- ^ Nagel, Z. D.; Klinman, J. P. (August 2006). "Tunneling and dynamics in enzymatic hydride transfer". Chemical Reviews. 106 (8): 3095–3118. doi:10.1021/cr050301x. PMID 16895320.
- ^ Pan, Ying-Hsi; Sader, Kasim; Powell, Jonathan J.; et al. (April 2009). "3D morphology of the human hepatic ferritin mineral core: New evidence for a subunit structure revealed by single particle analysis of HAADF-STEM images". Journal of Structural Biology. 166 (1): 22–31. doi:10.1016/j.jsb.2008.12.001. ISSN 1047-8477. PMC 2832756. PMID 19116170.
- ^ Wolszczak, Marian; Gajda, Joanna (2010-08-17). "Iron release from ferritin induced by light and ionizing radiation". Research on Chemical Intermediates. 36 (5): 549–563. doi:10.1007/s11164-010-0155-0. ISSN 0922-6168. S2CID 97639082.
- ^ Watt, G. D.; Jacobs, D.; Frankel, R. B. (October 1988). "Redox reactivity of bacterial and mammalian ferritin: is reductant entry into the ferritin interior a necessary step for iron release?". Proceedings of the National Academy of Sciences. 85 (20): 7457–7461. Bibcode:1988PNAS...85.7457W. doi:10.1073/pnas.85.20.7457. ISSN 0027-8424. PMC 282210. PMID 2845407.
- ^ Awschalom, D. D.; Smyth, J. F.; Grinstein, G.; DiVincenzo, D. P.; Loss, D. (1992-05-18). "Macroscopic quantum tunneling in magnetic proteins". Physical Review Letters. 68 (20): 3092–3095. Bibcode:1992PhRvL..68.3092A. doi:10.1103/physrevlett.68.3092. ISSN 0031-9007. PMID 10045605.
- ^ Xu, Degao; Watt, Gerald D.; Harb, John N.; Davis, Robert C. (2005-03-25). "Electrical Conductivity of Ferritin Proteins by Conductive AFM". Nano Letters. 5 (4): 571–577. Bibcode:2005NanoL...5..571X. doi:10.1021/nl048218x. ISSN 1530-6984. PMID 15826089.
- ^ Kumar, Karuppannan Senthil; Pasula, Rupali Reddy; Lim, Sierin; Nijhuis, Christian A. (2015-12-28). "Long-Range Tunneling Processes across Ferritin-Based Junctions". Advanced Materials. 28 (9): 1824–1830. doi:10.1002/adma.201504402. ISSN 0935-9648. PMID 26708136. S2CID 2238319.
- ^ Karuppannan, Senthil Kumar; Pasula, Rupali Reddy; Herng, Tun Seng; et al. (2021-05-20). "Room-temperature tunnel magnetoresistance across biomolecular tunnel junctions based on ferritin". Journal of Physics: Materials. 4 (3): 035003. Bibcode:2021JPhM....4c5003K. doi:10.1088/2515-7639/abfa79. hdl:10261/265689. ISSN 2515-7639. S2CID 235284565.
- ^ Mykhaylyk, Olga; Török, Gyula; Dudchenko, Olexandr; et al. (May 2004). "On the magnetic ordering of the iron storage proteins in tissues". Journal of Magnetism and Magnetic Materials. 272–276: 2422–2423. Bibcode:2004JMMM..272.2422M. doi:10.1016/j.jmmm.2003.12.472. ISSN 0304-8853.
- ^ Toolan, Daniel T. W.; Weir, Michael P.; Kilbride, Rachel C.; et al. (2020). "Controlling the structures of organic semiconductor–quantum dot nanocomposites through ligand shell chemistry". Soft Matter. 16 (34): 7970–7981. Bibcode:2020SMat...16.7970T. doi:10.1039/d0sm01109f. ISSN 1744-683X. PMID 32766663. S2CID 221075538.
- ^ Kouwenhoven, Leo P.; Marcus, Charles M.; McEuen, Paul L.; et al. (1997), "Electron Transport in Quantum Dots", Mesoscopic Electron Transport, Dordrecht: Springer Netherlands, pp. 105–214, doi:10.1007/978-94-015-8839-3_4, ISBN 978-90-481-4906-3, retrieved 2022-11-06
- ^ Yuan, Zhen; Atanassov, Plamen; Alsmadi, Abdel M.; et al. (2006-04-15). "Magnetic properties of self-assembled ferritin-core arrays". Journal of Applied Physics. 99 (8): 08Q509. Bibcode:2006JAP....99hQ509Y. doi:10.1063/1.2172546. ISSN 0021-8979.
- ^ Bera, Sudipta; Kolay, Jayeeta; Pramanik, Pallabi; et al. (2019). "Long-range solid-state electron transport through ferritin multilayers". Journal of Materials Chemistry C. 7 (29): 9038–9048. doi:10.1039/c9tc01744e. ISSN 2050-7526. S2CID 198849306.
- ^ Rourk, Christopher; Huang, Yunbo; Chen, Minjing; Shen, Cai (2021-08-12). "Indication of Strongly Correlated Electron Transport and Mott Insulator in Disordered Multilayer Ferritin Structures (DMFS)". Materials. 14 (16): 4527. Bibcode:2021Mate...14.4527R. doi:10.3390/ma14164527. ISSN 1996-1944. PMC 8399281. PMID 34443050.
- ^ Labra-Muñoz, Jacqueline A.; de Reuver, Arie; Koeleman, Friso; Huber, Martina; van der Zant, Herre S. J. (2022-05-15). "Ferritin-Based Single-Electron Devices". Biomolecules. 12 (5): 705. doi:10.3390/biom12050705. ISSN 2218-273X. PMC 9138424. PMID 35625632.
- ^ Zulu, Michael Z.; Martinez, Fernando O.; Gordon, Siamon; Gray, Clive M. (2019). "The Elusive Role of Placental Macrophages: The Hofbauer Cell". Journal of Innate Immunity. 11 (6): 447–456. doi:10.1159/000497416. ISSN 1662-811X. PMC 6758944. PMID 30970346. S2CID 108293800.
- ^ Rourk, Christopher J. (May 2019). "Indication of quantum mechanical electron transport in human substantia nigra tissue from conductive atomic force microscopy analysis". Biosystems. 179: 30–38. Bibcode:2019BiSys.179...30R. doi:10.1016/j.biosystems.2019.02.003. ISSN 0303-2647. PMID 30826349. S2CID 73509918.
- ^ Friedrich, I.; Reimann, K.; Jankuhn, S.; et al. (2021-03-22). "Cell specific quantitative iron mapping on brain slices by immuno-µPIXE in healthy elderly and Parkinson's disease". Acta Neuropathologica Communications. 9 (1): 47. doi:10.1186/s40478-021-01145-2. ISSN 2051-5960. PMC 7986300. PMID 33752749.
- ^ Sulzer, David; Cassidy, Clifford; Horga, Guillermo; et al. (2018-04-10). "Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease". npj Parkinson's Disease. 4 (1): 11. doi:10.1038/s41531-018-0047-3. ISSN 2373-8057. PMC 5893576. PMID 29644335. S2CID 4736738.
- ^ Xiong, Nian; Huang, Jinsha; Zhang, Zhentao; et al. (2009-11-18). "Stereotaxical Infusion of Rotenone: A Reliable Rodent Model for Parkinson's Disease". PLOS ONE. 4 (11): e7878. Bibcode:2009PLoSO...4.7878X. doi:10.1371/journal.pone.0007878. ISSN 1932-6203. PMC 2774159. PMID 19924288.
- ^ Muirden, K. D. (1966-09-01). "Ferritin in Synovial Cells in Patients with Rheumatoid Arthritis". Annals of the Rheumatic Diseases. 25 (5): 387–401. doi:10.1136/ard.25.5.387. ISSN 0003-4967. PMC 2453468. PMID 4161916.
- ^ ORINO, Kouichi; LEHMAN, Lori; TSUJI, Yoshiaki; et al. (2001-06-25). "Ferritin and the response to oxidative stress". Biochemical Journal. 357 (1): 241–247. doi:10.1042/bj3570241. ISSN 0264-6021. PMC 1221947. PMID 11415455.
- ^ Aronova, Maria A.; Noh, Seung-Jae; Zhang, Guofeng; Byrnes, Colleen; Meier, Emily Riehm; Kim, Young C.; Leapman, Richard D. (August 2021). "Use of dual-electron probes reveals the role of ferritin as an iron depot in ex vivo erythropoiesis". iScience. 24 (8): 102901. Bibcode:2021iSci...24j2901A. doi:10.1016/j.isci.2021.102901. ISSN 2589-0042. PMC 8355919. PMID 34401678.
- ^ Garg, Mayank; Vishwakarma, Neelam; Sharma, Amit L.; Singh, Suman (2021-07-08). "Amine-Functionalized Graphene Quantum Dots for Fluorescence-Based Immunosensing of Ferritin". ACS Applied Nano Materials. 4 (7): 7416–7425. doi:10.1021/acsanm.1c01398. ISSN 2574-0970. S2CID 237804893.
- ^ Klopping, H. L. (May 1971). "Olfactory theories and the odors of small molecules". Journal of Agricultural and Food Chemistry. 19 (5): 999–1004. doi:10.1021/jf60177a002. PMID 5134656.
- ^ Dyson, Malcolm G. (1938-07-09). "The scientific basis of odour". Journal of the Society of Chemical Industry. 57 (28): 647–651. doi:10.1002/jctb.5000572802.
- ^ Jump up to:a b c Turin, L. (December 1996). "A spectroscopic mechanism for primary olfactory reception". Chemical Senses. 21 (6): 773–791. doi:10.1093/chemse/21.6.773. PMID 8985605.
- ^ "Odorant shape and vibration likely lead to olfaction satisfaction". Retrieved 2018-11-08.
- ^ Buck, Linda; Axel, Richard (April 5, 1991). "A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition" (PDF). Cell. 65 (1): 175–187. doi:10.1016/0092-8674(91)90418-x. PMID 1840504. Archived from the original on 29 August 2017. Retrieved November 7, 2018.
- ^ Block, Eric; Batista, Victor S.; Matsunami, Hiroaki; Zhuang, Hanyi; Ahmed, Lucky (May 2017). "The role of metals in mammalian olfaction of low molecular weight organosulfur compounds". Natural Product Reports. 34 (5): 529–557. doi:10.1039/c7np00016b. PMC 5542778. PMID 28471462.
- ^ Keller, A.; Vosshall, L. B. (April 2004). "A psychophysical test of the vibration theory of olfaction". Nature Neuroscience. 7 (4): 337–338. doi:10.1038/nn1215. PMID 15034588. S2CID 1073550.
- ^ Johnson, P. J. M.; Farag, M. H.; Halpin, A.; et al. (April 2017). "The Primary Photochemistry of Vision Occurs at the Molecular Speed Limit" (PDF). The Journal of Physical Chemistry B. 121 (16): 4040–4047. doi:10.1021/acs.jpcb.7b02329. PMID 28358485. S2CID 4837083.
- ^ Schoenlein, R. W.; Peteanu, L. A.; Mathies, R. A.; Shank, C. V. (October 1991). "The first step in vision: femtosecond isomerization of rhodopsin". Science. 254 (5030): 412–415. Bibcode:1991Sci...254..412S. doi:10.1126/science.1925597. PMID 1925597.
- ^ Gibbs, Philip. "The Human Eye and Single Photons". math.ucr.edu. Retrieved 2018-11-05.
- ^ Loulakis, M.; Blatsios, G.; Vrettou, C. S.; Kominis, I. K. (2017). "Quantum Biometrics with Retinal Photon Counting". Physical Review Applied. 8 (4): 044012. arXiv:1704.04367. Bibcode:2017PhRvP...8d4012L. doi:10.1103/PhysRevApplied.8.044012. S2CID 119256067.
- ^ Emerging Technology from the arXiv. "The unique way your eyes detect photons could be used to guarantee your identity, say physicists". MIT Technology Review. Retrieved 2018-11-08.
- ^ Mathis, Paul (December 2000). "Photosynthesis by D.O. Hall and K.K. Rao, Cambridge, Cambridge, CB2 2RU, U.K., ISBN 0 521 64257 4, £ 35, University Press". Plant Science. 160 (1): 179–180. doi:10.1016/s0168-9452(00)00371-x. ISSN 0168-9452.
- ^ Sumi, Hitoshi (January 2001). "Bacterial photosynthesis begins with quantum-mechanical coherence". The Chemical Record. 1 (6): 480–493. doi:10.1002/tcr.10004. ISSN 1527-8999. PMID 11933253.
- ^ Lokstein, Heiko; Renger, Gernot; Götze, Jan P. (January 2021). "Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions". Molecules. 26 (11): 3378. doi:10.3390/molecules26113378. ISSN 1420-3049. PMC 8199901. PMID 34204994.
- ^ Dostál, Jakub; Mančal, Tomáš; Augulis, Ramūnas; Vácha, František; Pšenčík, Jakub; Zigmantas, Donatas (July 2012). "Two-dimensional electronic spectroscopy reveals ultrafast energy diffusion in chlorosomes". Journal of the American Chemical Society. 134 (28): 11611–11617. doi:10.1021/ja3025627. PMID 22690836.
- ^ Keren, Nir; Paltiel, Yossi (June 2018). "Photosynthetic Energy Transfer at the Quantum/Classical Border". Trends in Plant Science. 23 (6): 497–506. doi:10.1016/j.tplants.2018.03.007. ISSN 1360-1385. PMID 29625851. S2CID 4644544.
- ^ Kassal, Ivan; Yuen-Zhou, Joel; Rahimi-Keshari, Saleh (2013-02-07). "Does Coherence Enhance Transport in Photosynthesis?". The Journal of Physical Chemistry Letters. 4 (3): 362–367. arXiv:1210.5022. doi:10.1021/jz301872b. ISSN 1948-7185. PMID 26281724. S2CID 7074341.
- ^ Keren, Nir; Paltiel, Yossi (June 2018). "Photosynthetic Energy Transfer at the Quantum/Classical Border". Trends in Plant Science. 23 (6): 497–506. doi:10.1016/j.tplants.2018.03.007. ISSN 1360-1385. PMID 29625851. S2CID 4644544.
- ^ Engel, Gregory S.; Calhoun, Tessa R.; Read, Elizabeth L.; Ahn, Tae-Kyu; Mančal, Tomáš; Cheng, Yuan-Chung; et al. (April 2007). "Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems" (PDF). Nature. 446 (7137): 782–786. Bibcode:2007Natur.446..782E. doi:10.1038/nature05678. PMID 17429397. S2CID 13865546.
- ^ Collini, Elisabetta; Wong, Cathy Y.; Wilk, Krystyna E.; Curmi, Paul M. G.; Brumer, Paul; Scholes, Gregory D. (February 2010). "Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature". Nature. 463 (7281): 644–647. Bibcode:2010Natur.463..644C. doi:10.1038/nature08811. PMID 20130647. S2CID 4369439.
- ^ Tempelaar, R.; Jansen, T. L. C.; Knoester, J. (November 2014). "Vibrational beatings conceal evidence of electronic coherence in the FMO light-harvesting complex". The Journal of Physical Chemistry B. 118 (45): 12865–12872. doi:10.1021/jp510074q. PMID 25321492.
- ^ Christensson, Niklas; Kauffmann, Harald F.; Pullerits, Tõnu; Mančal, Tomáš (June 2012). "Origin of long-lived coherences in light-harvesting complexes". The Journal of Physical Chemistry B. 116 (25): 7449–7454. arXiv:1201.6325. Bibcode:2012arXiv1201.6325C. doi:10.1021/jp304649c. PMC 3789255. PMID 22642682.
- ^ Butkus, Vytautas; Zigmantas, Donatas; Valkunas, Leonas; Abramavicius, Darius (2012). "Vibrational vs. electronic coherences in 2D spectrum of molecular systems". Chemical Physics Letters. 545: 40–43. arXiv:1201.2753. Bibcode:2012CPL...545...40B. doi:10.1016/j.cplett.2012.07.014. ISSN 0009-2614. S2CID 96663719.
- ^ Tiwari, Vivek; Peters, William K.; Jonas, David M. (2012-12-24). "Electronic resonance with anticorrelated pigment vibrations drives photosynthetic energy transfer outside the adiabatic framework". Proceedings of the National Academy of Sciences. 110 (4): 1203–1208. doi:10.1073/pnas.1211157110. ISSN 0027-8424. PMC 3557059. PMID 23267114.
- ^ Thyrhaug, Erling; Žídek, Karel; Dostál, Jakub; Bína, David; Zigmantas, Donatas (May 2016). "Exciton Structure and Energy Transfer in the Fenna-Matthews-Olson Complex". The Journal of Physical Chemistry Letters. 7 (9): 1653–1660. doi:10.1021/acs.jpclett.6b00534. PMID 27082631. S2CID 26355154.
- ^ Fujihashi, Yuta; Fleming, Graham R.; Ishizaki, Akihito (June 2015). "Impact of environmentally induced fluctuations on quantum mechanically mixed electronic and vibrational pigment states in photosynthetic energy transfer and 2D electronic spectra". The Journal of Chemical Physics. 142 (21): 212403. arXiv:1505.05281. Bibcode:2015JChPh.142u2403F. doi:10.1063/1.4914302. PMID 26049423. S2CID 1082742.
- ^ Jump up to:a b Marais, Adriana; Adams, Betony; Ringsmuth, Andrew K.; et al. (November 2018). "The future of quantum biology". Journal of the Royal Society, Interface. 15 (148): 20180640. doi:10.1098/rsif.2018.0640. PMC 6283985. PMID 30429265.
- ^ Mohseni, Masoud; Rebentrost, Patrick; Lloyd, Seth; Aspuru-Guzik, Alán (November 2008). "Environment-assisted quantum walks in photosynthetic energy transfer". The Journal of Chemical Physics. 129 (17): 174106. arXiv:0805.2741. Bibcode:2008JChPh.129q4106M. doi:10.1063/1.3002335. PMID 19045332. S2CID 938902.
- ^ Plenio, M. B.; Huelga, S. F. (2008-11-01). "Dephasing-assisted transport: quantum networks and biomolecules – IOPscience". New Journal of Physics. 10 (11): 113019. arXiv:0807.4902. Bibcode:2008NJPh...10k3019P. doi:10.1088/1367-2630/10/11/113019. S2CID 12172391.
- ^ Lloyd, S. (2014-03-10). Optimal Energy Transport in Photosynthesis (Speech). From Atomic to Mesoscale: The Role of Quantum Coherence in Systems of Various Complexities. Institute for Theoretical, Atomic and Molecular and Optical Physics, Harvard-Smithsonian Center for Astrophysics, Cambridge, Massachusetts. Retrieved 2019-09-30.
- ^ Lee, H. (2009). "Quantum Coherence Accelerating Photosynthetic Energy Transfer". Ultrafast Phenomena XVI. Springer Series in Chemical Physics. Vol. 92. pp. 607–609. Bibcode:2009up16.book..607L. doi:10.1007/978-3-540-95946-5_197. ISBN 978-3-540-95945-8. Archived from the original on February 3, 2021.
- ^ Walschaers, Mattia; Fernandez-de-Cossio Diaz, Jorge; Mulet, Roberto; Buchleitner, Andreas (November 2013). "Optimally designed quantum transport across disordered networks". Physical Review Letters. 111 (18): 180601. arXiv:1207.4072. Bibcode:2013PhRvL.111r0601W. doi:10.1103/PhysRevLett.111.180601. PMID 24237498. S2CID 40710862.
- ^ Halpin, A.; Johnson, P. J. M.; Tempelaar, R.; Murphy, R. S.; Knoester, J.; Jansen, T. L. C.; Miller, R. J. D. (March 2014). "Two-dimensional spectroscopy of a molecular dimer unveils the effects of vibronic coupling on exciton coherences". Nature Chemistry. 6 (3): 196–201. Bibcode:2014NatCh...6..196H. doi:10.1038/nchem.1834. PMID 24557133. S2CID 5059005.
- ^ Duan, H. -G.; Prokhorenko, V. I.; Cogdell, R.; Ashraf, K.; Stevens, A. L.; Thorwart, M.; Miller, R. J. D. (August 2017). "Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer". Proceedings of the National Academy of Sciences of the United States of America. 114 (32): 8493–8498. arXiv:1610.08425. Bibcode:2017PNAS..114.8493D. doi:10.1073/pnas.1702261114. PMC 5559008. PMID 28743751.
- ^ Cao, Jianshu; Cogdell, Richard J.; Coker, David F.; et al. (April 2020). "Quantum biology revisited". Science Advances. 6 (14): eaaz4888. Bibcode:2020SciA....6.4888C. doi:10.1126/sciadv.aaz4888. PMC 7124948. PMID 32284982.
- ^ Huelga, S. F.; Plenio, M. B. (2013-07-01). "Vibrations, quanta and biology". Contemporary Physics. 54 (4): 181–207. arXiv:1307.3530. Bibcode:2013ConPh..54..181H. doi:10.1080/00405000.2013.829687. ISSN 0010-7514. S2CID 15030104.
- ^ DeVault, D.; Chance, B. (November 1966). "Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling". Biophysical Journal. 6 (6): 825–847. Bibcode:1966BpJ.....6..825D. doi:10.1016/S0006-3495(66)86698-5. PMC 1368046. PMID 5972381.
- ^ Jump up to:a b Morowitz, H. (1968). Energy Flow in Biology. New York and London: Academic Press. pp. 55–56, 103–105, 116.
- ^ Hayashi, Tomoyuki; Stuchebrukhov, Alexei A. (2011-05-12). "Quantum Electron Tunneling in Respiratory Complex I". The Journal of Physical Chemistry B. 115 (18): 5354–5364. doi:10.1021/jp109410j. ISSN 1520-6106. PMC 4230448. PMID 21495666.
- ^ Hagras, Muhammad A.; Hayashi, Tomoyuki; Stuchebrukhov, Alexei A. (2015-11-19). "Quantum Calculations of Electron Tunneling in Respiratory Complex III". The Journal of Physical Chemistry B. 119 (46): 14637–14651. doi:10.1021/acs.jpcb.5b09424. ISSN 1520-6106. PMID 26505078.
- ^ Bennett, James P.; Onyango, Isaac G. (February 2021). "Energy, Entropy and Quantum Tunneling of Protons and Electrons in Brain Mitochondria: Relation to Mitochondrial Impairment in Aging-Related Human Brain Diseases and Therapeutic Measures". Biomedicines. 9 (2): 225. doi:10.3390/biomedicines9020225. ISSN 2227-9059. PMC 7927033. PMID 33671585.
- ^ "Quantum Theory", A Unified Grand Tour of Theoretical Physics, 2nd edition, Taylor & Francis, pp. 123–145, 2015-05-05, doi:10.1201/noe0750306041-9, ISBN 978-0-429-10829-7, retrieved 2023-11-08
- ^ Arndt, Markus; Juffmann, Thomas; Vedral, Vlatko (December 2009). "Quantum physics meets biology". HFSP Journal. 3 (6): 386–400. arXiv:0911.0155. doi:10.2976/1.3244985. PMC 2839811. PMID 20234806.
- ^ Jump up to:a b Davies, P. C. (January 2008). "A quantum origin of life?". Quantum aspects of life. Imperial College Press. pp. 3–18. doi:10.1142/9781848162556_0001. ISBN 978-1-84816-253-2.
- ^ Davydov, Alexander S. (1973). "The theory of contraction of proteins under their excitation". Journal of Theoretical Biology. 38 (3): 559–569. Bibcode:1973JThBi..38..559D. doi:10.1016/0022-5193(73)90256-7. PMID 4266326.
- ^ Davydov, Alexander S. (1977). "Solitons and energy transfer along protein molecules". Journal of Theoretical Biology. 66 (2): 379–387. Bibcode:1977JThBi..66..379D. doi:10.1016/0022-5193(77)90178-3. PMID 886872.
- ^ Davydov, Alexander S. (1982). "Solitons in quasi-one-dimensional molecular structures". Soviet Physics Uspekhi. 25 (12): 898–918. doi:10.1070/pu1982v025n12abeh005012.
- ^ Scott, Alwyn C. (1985). "Davydov solitons in polypeptides". Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences. 315 (1533): 423–436. Bibcode:1985RSPTA.315..423S. doi:10.1098/rsta.1985.0049. S2CID 86823456.
- ^ Hore, Peter J.; Mouritsen, Henrik (April 2022). "The Quantum Nature of Bird Migration". Scientific American: 24–29.
- ^ Jump up to:a b c Hore, P. J.; Mouritsen, Henrik (July 2016). "The Radical-Pair Mechanism of Magnetoreception". Annual Review of Biophysics. 45 (1): 299–344. doi:10.1146/annurev-biophys-032116-094545. PMID 27216936. S2CID 7099782.
- ^ Schulten, K.; Swenberg, C. E.; Weller, A. (1978). "A Biomagnetic Sensory Mechanism Based on Magnetic Field Modulated Coherent Electron Spin Motion : Zeitschrift für Physikalische Chemie". Zeitschrift für Physikalische Chemie. 111: 1–5. doi:10.1524/zpch.1978.111.1.001. S2CID 124644286.
- ^ Kominis, I. K. (2015). "The radical-pair mechanism as a paradigm for the emerging science of quantum biology". Modern Physics Letters B. 29: 1530013. arXiv:1512.00450. Bibcode:2015MPLB...29S0013K. doi:10.1142/S0217984915300136. S2CID 119276673.
- ^ Rodgers, C. T. (2009-01-01). "Magnetic field effects in chemical systems". Pure and Applied Chemistry. 81 (1): 19–43. doi:10.1351/PAC-CON-08-10-18. ISSN 1365-3075.
- ^ Steiner, U. E.; Ulrich, T. (1989-01-01). "Magnetic field effects in chemical kinetics and related phenomena". Chemical Reviews. 89 (1): 51–147. doi:10.1021/cr00091a003. ISSN 0009-2665.
- ^ Woodward, J. R. (2002-09-01). "Radical Pairs in Solution". Progress in Reaction Kinetics and Mechanism. 27 (3): 165–207. doi:10.3184/007967402103165388. S2CID 197049448.
- ^ Jump up to:a b Wiltschko, Roswitha; Ahmad, Margaret; Nießner, Christine; Gehring, Dennis; Wiltschko, Wolfgang (May 2016). "Light-dependent magnetoreception in birds: the crucial step occurs in the dark". Journal of the Royal Society, Interface. 13 (118): 20151010. doi:10.1098/rsif.2015.1010. PMC 4892254. PMID 27146685.
- ^ Xu, Jingjing; Jarocha, Lauren E.; Zollitsch, Tilo; et al. (2021-06-24). "Magnetic sensitivity of cryptochrome 4 from a migratory songbird". Nature. 594 (7864): 535–540. Bibcode:2021Natur.594..535X. doi:10.1038/s41586-021-03618-9. ISSN 0028-0836. PMID 34163056. S2CID 235625675.
- ^ "DNA and Mutations". evolution.berkeley.edu. Retrieved 2018-11-05.
- ^ Jump up to:a b Trixler, F. (August 2013). "Quantum Tunnelling to the Origin and Evolution of Life". Current Organic Chemistry. 17 (16): 1758–1770. doi:10.2174/13852728113179990083. PMC 3768233. PMID 24039543.
- ^ Slocombe, L.; Al-Khalili, Jim S.; Sacchi, M. (February 2021). "Quantum and classical effects in DNA point mutations: Watson-Crick tautomerism in AT and GC base pairs". Physical Chemistry Chemical Physics. 23 (7): 4141–4150. Bibcode:2021PCCP...23.4141S. doi:10.1039/D0CP05781A. PMID 33533770.
- ^ Slocombe, Louie; Sacchi, Marco; Al-Khalili, Jim (2022-05-05). "An open quantum systems approach to proton tunnelling in DNA". Communications Physics. 5 (1): 109. arXiv:2110.00113. Bibcode:2022CmPhy...5..109S. doi:10.1038/s42005-022-00881-8. ISSN 2399-3650. S2CID 238253421.
- ^ Yu, S. L.; Lee, S. K. (March 2017). "Ultraviolet radiation: DNA damage, repair, and human disorders". Molecular & Cellular Toxicology. 13 (1): 21–28. doi:10.1007/s13273-017-0002-0. S2CID 27532980.
- ^ Levine, R. D. (2005). Molecular Reaction Dynamics. Cambridge University Press. pp. 16–18. ISBN 978-0-521-84276-1.
- ^ Krug, Harald; Brune, Harald; Schmid, Gunter; et al. (2006). Nanotechnology: Assessment and Perspectives. Springer-Verlag. pp. 197–240. ISBN 978-3-540-32819-3.
- ^ Al-Khalili, Jim; Lilliu, Samuele (2020). "Quantum biology". Scientific Video Protocols. 1 (1): 1–4. doi:10.32386/scivpro.000020. S2CID 240530837.
- ^ Bier, Martin (2010). "Quantum Consciousness and Other Spooky Myths" (PDF). Skeptic: 40–43.
External links[edit]
Philip Ball (2015). "Quantum Biology: An Introduction". The Royal Institution
Quantum Biology and the Hidden Nature of Nature, World Science Festival 2012, video of podium discussion
Quantum Biology: Current Status and Opportunities, September 17-18, 2012, University of Surrey, UK
This page was last edited on 18 March 2024, at 22:48 (UT
==
Quantum physics proposes a new way to study biology – and the results could revolutionize our understanding of how life works
Published: May 15, 2023 10.33pm AEST
Author
Quantum Biology Tech (QuBiT) Lab, Assistant Professor of Electrical and Computer Engineering, University of California, Los Angeles
Disclosure statement
Clarice D. Aiello receives funding from NSF, ONR, IDOR Foundation, Faggin Foundation, Templeton Foundation.
Partners
University of California, Los Angeles provides funding as a member of The Conversation US.
View all partners
Imagine using your cellphone to control the activity of your own cells to treat injuries and disease. It sounds like something from the imagination of an overly optimistic science fiction writer. But this may one day be a possibility through the emerging field of quantum biology.
Over the past few decades, scientists have made incredible progress in understanding and manipulating biological systems at increasingly small scales, from protein folding to genetic engineering. And yet, the extent to which quantum effects influence living systems remains barely understood.
Quantum effects are phenomena that occur between atoms and molecules that can’t be explained by classical physics. It has been known for more than a century that the rules of classical mechanics, like Newton’s laws of motion, break down at atomic scales. Instead, tiny objects behave according to a different set of laws known as quantum mechanics.
Quantum mechanics describes the properties of atoms and molecules.
For humans, who can only perceive the macroscopic world, or what’s visible to the naked eye, quantum mechanics can seem counterintuitive and somewhat magical. Things you might not expect happen in the quantum world, like electrons “tunneling” through tiny energy barriers and appearing on the other side unscathed, or being in two different places at the same time in a phenomenon called superposition.
Make better decisions - find out what the experts think.Get newsletter
I am trained as a quantum engineer. Research in quantum mechanics is usually geared toward technology. However, and somewhat surprisingly, there is increasing evidence that nature – an engineer with billions of years of practice – has learned how to use quantum mechanics to function optimally. If this is indeed true, it means that our understanding of biology is radically incomplete. It also means that we could possibly control physiological processes by using the quantum properties of biological matter.
Quantumness in biology is probably real
Researchers can manipulate quantum phenomena to build better technology. In fact, you already live in a quantum-powered world: from laser pointers to GPS, magnetic resonance imaging and the transistors in your computer – all these technologies rely on quantum effects.
In general, quantum effects only manifest at very small length and mass scales, or when temperatures approach absolute zero. This is because quantum objects like atoms and molecules lose their “quantumness” when they uncontrollably interact with each other and their environment. In other words, a macroscopic collection of quantum objects is better described by the laws of classical mechanics. Everything that starts quantum dies classical. For example, an electron can be manipulated to be in two places at the same time, but it will end up in only one place after a short while – exactly what would be expected classically.
Electrons can be in two places at the same time, but will end up in one location eventually.
In a complicated, noisy biological system, it is thus expected that most quantum effects will rapidly disappear, washed out in what the physicist Erwin Schrödinger called the “warm, wet environment of the cell.” To most physicists, the fact that the living world operates at elevated temperatures and in complex environments implies that biology can be adequately and fully described by classical physics: no funky barrier crossing, no being in multiple locations simultaneously.
Chemists, however, have for a long time begged to differ. Research on basic chemical reactions at room temperature unambiguously shows that processes occurring within biomolecules like proteins and genetic material are the result of quantum effects. Importantly, such nanoscopic, short-lived quantum effects are consistent with driving some macroscopic physiological processes that biologists have measured in living cells and organisms. Research suggests that quantum effects influence biological functions, including regulating enzyme activity, sensing magnetic fields, cell metabolism and electron transport in biomolecules.
How to study quantum biology
The tantalizing possibility that subtle quantum effects can tweak biological processes presents both an exciting frontier and a challenge to scientists. Studying quantum mechanical effects in biology requires tools that can measure the short time scales, small length scales and subtle differences in quantum states that give rise to physiological changes – all integrated within a traditional wet lab environment.
In my work, I build instruments to study and control the quantum properties of small things like electrons. In the same way that electrons have mass and charge, they also have a quantum property called spin. Spin defines how the electrons interact with a magnetic field, in the same way that charge defines how electrons interact with an electric field. The quantum experiments I have been building since graduate school, and now in my own lab, aim to apply tailored magnetic fields to change the spins of particular electrons.
Research has demonstrated that many physiological processes are influenced by weak magnetic fields. These processes include stem cell development and maturation, cell proliferation rates, genetic material repair and countless others. These physiological responses to magnetic fields are consistent with chemical reactions that depend on the spin of particular electrons within molecules. Applying a weak magnetic field to change electron spins can thus effectively control a chemical reaction’s final products, with important physiological consequences.
Birds use quantum effects in navigation.
Currently, a lack of understanding of how such processes work at the nanoscale level prevents researchers from determining exactly what strength and frequency of magnetic fields cause specific chemical reactions in cells. Current cellphone, wearable and miniaturization technologies are already sufficient to produce tailored, weak magnetic fields that change physiology, both for good and for bad. The missing piece of the puzzle is, hence, a “deterministic codebook” of how to map quantum causes to physiological outcomes.
In the future, fine-tuning nature’s quantum properties could enable researchers to develop therapeutic devices that are noninvasive, remotely controlled and accessible with a mobile phone. Electromagnetic treatments could potentially be used to prevent and treat disease, such as brain tumors, as well as in biomanufacturing, such as increasing lab-grown meat production.
A whole new way of doing science
Quantum biology is one of the most interdisciplinary fields to ever emerge. How do you build community and train scientists to work in this area?
Since the pandemic, my lab at the University of California, Los Angeles and the University of Surrey’s Quantum Biology Doctoral Training Centre have organized Big Quantum Biology meetings to provide an informal weekly forum for researchers to meet and share their expertise in fields like mainstream quantum physics, biophysics, medicine, chemistry and biology.
Research with potentially transformative implications for biology, medicine and the physical sciences will require working within an equally transformative model of collaboration. Working in one unified lab would allow scientists from disciplines that take very different approaches to research to conduct experiments that meet the breadth of quantum biology from the quantum to the molecular, the cellular and the organismal.
The existence of quantum biology as a discipline implies that traditional understanding of life processes is incomplete. Further research will lead to new insights into the age-old question of what life is, how it can be controlled and how to learn with nature to build better quantum technologies.
Physics
Quantum mechanics
Quantum
Quantum physics
Electromagnetic radiation
Electrons
Biology
Magnetic fields
Science
atomic scale
Quantum technologies
Biophysics
Subatomic particles
Interdisciplinary research
Quantum science
Cellphones
Quantum superposition
Electron
Electromagnetic energy
Quantum technology
Magnetic field
Science collaboration
Interdisciplinary science
Ditch the algorithms for a more constructive inbox.
Soon, when you type a question into a search engine, the first result may be an AI-generated answer sourced from the open web. There is every chance the answers presented by the algorithms that shape our contemporary reality will be based on disinformation and misinformation. But here at The Conversation our articles are written by academics and edited by professional journalists. Sign-up to our FREE daily newsletter to have your burning questions answered by bona-fide experts.
Get newsletter
Misha Ketchell
Editor
