Vol. 126, No. 6FocusOpen Access
What Is Your Gut Telling You? Exploring the Role of the Microbiome in Gut–Brain Signaling
Lindsey Konkel
Published:6 June 2018CID: 062001https://doi.org/10.1289/EHP3127Cited by:1
View Article in:中文版
Sections
Tools
Share
On 6 June 1822, French Canadian fur trade voyageur Alexis St. Martin was shot accidentally in the stomach at an American Fur Company store on Michigan’s Mackinac Island.1 The blast left a gaping wound in St. Martin’s abdomen. St. Martin eventually recovered from the gruesome accident, but the wound never closed completely, leaving a small permanent opening in his stomach wall.1
His surgeon, William Beaumont, began monitoring gastric secretions through this opening in St. Martin’s body. Beaumont, who would later become known as the father of gastric physiology, would attach various types of food to a string and suspend them through the hole. Later he would pull out the string to see what portion of the food had been digested. During these experiments, Beaumont noticed that St. Martin’s mood seemed to affect how quickly he digested food. When St. Martin was irritable, for instance, food broke down more slowly.2
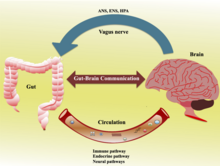
These early observations provided the first clues of crosstalk between the brain and the gut. Researchers later called this bidirectional communication system the gut–brain axis. Over the years, studies have revealed that the brain influences the gastrointestinal (GI) tract through several mechanisms that involve the nervous and immune systems.2

From the moment of birth—and possibly even earlier—our microbiomes begin to develop. There is evidence that a healthy gut microbiome is important for brain development, but as toxicologist Matt Rand explains, “the complexity of the microbiome, with many thousands of microbial species existing as a community, predicts that individual ‘superbugs’ are not likely to be found that single-handedly mediate a gut–brain benefit or detriment.” Image: © Andesign101/iStockphoto.
Only recently have scientists recognized the importance of a third component to the gut–brain axis: the trillions of bacteria, viruses, archaea, and eukaryotes that make up the gut microbiome. In little more than a decade, researchers have uncovered intriguing associations between gut bacteria and a host of neurological disorders and psychiatric conditions. These include depression, anxiety, autism spectrum disorders (ASDs), and Parkinson’s disease.2
Most of the early research on the microbiome–gut–brain axis has been conducted in rodents.3 Germ-free mice—which are born in sterile conditions and free of all microorganisms—are popular for gut flora research because scientists can inoculate the mice with specific microbes and watch what happens.
Now, additional researchers are beginning to probe the connection in humans. Outside neuroscience, gut microbiome research in laboratory animals and humans is changing the way some environmental health scientists view the effects of environmental exposures on neurodevelopment and brain chemistry.
Developmental Links
Microbes colonize the infant gut shortly after birth. Starting with delivery2—and possibly even earlier in the womb4,5—infants are inoculated with microorganisms from their mother’s body. These pioneering microbes play a critical role in shaping the development of the GI tract and immune system.2 They also set the basis for the adult microbiome. By the end of the first 3–5 years of life, a child’s gut flora closely resemble those of an adult.2 “The architecture of the gut microbiome, once established in the first few years, remains more or less stable for life,” says Emeran Mayer, a gastroenterologist and neuroscientist at the University of California, Los Angeles.
This critical window for microbes to colonize the infant gut coincides with a period of rapid brain development. A study published in 2004 provided the first experimental evidence that the two domains could be related.6 A group of scientists led by Nobuyuki Sudo of Kyushu University in Japan showed that germ-free laboratory mice inoculated early in life with a probiotic (i.e., beneficial) gut bacterial strain were less likely than conventionally reared mice to exhibit anxiety-like behaviors in stressful environments, such as mazes, brightly lit boxes, and open spaces.7,8,9
Beyond the critical early life window, some studies have shown that germ-free mice colonized with probiotics during adulthood also are less likely to engage in some anxiety- and depression-like behaviors.10 In one study, germ-free mice did not join in normal social behaviors and engaged in more repetitive behaviors than their conventionally colonized counterparts.11 However, in another study using a rodent model of autism, researchers showed it was possible to reverse deficits in social interactions by colonizing the initially germ-free animals with the beneficial bacterium Bacteroides fragilis.12
Some of the microbiome’s influence on neurodevelopment might be sex-specific. In a 2013 study, researchers showed that manipulating the microbiome resulted in altered levels of the neurotransmitter serotonin in male germ-free mice but not in females.9
Overall, accumulating evidence from rodent studies suggests links among gut flora, brain biochemistry, and behavior.10 Many of the findings remain untested in humans, however, and although the germ-free mouse is a powerful tool for testing hypotheses about commensal bacteria, it is not always environmentally relevant,10 because humans are bathed in microbes from birth. “In humans, you are looking for subtle variations in what bugs are present and what they are doing,” says Rebecca Knickmeyer, a neuroscientist at the University of North Carolina at Chapel Hill.
Researchers have suspected a relationship between microbial colonization after birth and brain development, but until recently, no empirical evidence in humans has been found that demonstrates the link.13 That’s starting to change as researchers take what they have learned in early studies of germ-free mice and begin testing hypotheses in people. “Ultimately, the goal would be to manipulate the microbiome to improve cognitive functioning and reduce the risk for developmental and later-life disorders,” says Knickmeyer.

Germ-free mice are well suited for microbiome research because they can be selectively inoculated with microbes of interest. Experiments with germ-free mice have yielded intriguing clues about the possible influence of the gut microbiome on behavior and neurodevelopment. However, it is still unclear whether these findings are relevant to humans. Image: © muratsenel/iStockphoto.
Recently Knickmeyer et al. took the first steps toward translating animal data to the clinic by linking the composition of an infant’s intestinal flora to its cognitive development.13 The researchers collected fecal samples from 89 typically developing 1-year-olds. They compared the microbial composition of the stool—a technique used to identify gut bacteria—to cognitive outcomes on an early learning test and magnetic resonance imaging scans of the brain at ages 1 and 2 years.
Knickmeyer’s group found that children with the highest levels of gut microbial diversity scored lower on tests of expressive language (how a person communicates their needs and wants) and visual perceptual processing (how the brain makes sense of what the eyes see), in comparison with children who had a less diverse gut microbiome.
The findings, says Knickmeyer, were a bit unexpected, because having a diverse microbiome is typically seen as a benefit. The thinking is that having many different kinds of bacteria in the gut can help buffer against environmental perturbations that could shift gut microbial composition away from its equilibrium, she explains.
The researchers are not sure why having a more diverse microbiome, with a more adultlike profile of constituent microorganisms, was associated with worse cognitive outcomes. One possibility is that children with more microbial diversity could be harboring harmful bacterial strains, says Knickmeyer.
The researchers also do not know whether the microbiome itself actually caused the differences in cognitive scores. Instead, it could be acting as a proxy for some other environmental or genetic factor that’s driving the association, or there could be some other explanation altogether. It will be important to confirm these findings in other populations.
Erika Claud is using animal research to test associations seen in her work as a neonatologist at the University of Chicago Medical Center. Her research focuses on necrotizing enterocolitis (NEC), an inflammatory bowel disorder that affects premature infants. In babies with NEC, disease-causing bacteria invade the intestinal wall, causing perforations that can result in a life-threatening infection.14
Earlier comparisons of preterm babies with and without NEC found that babies with the condition were more likely to have poorer neurodevelopmental outcomes.15,16,17 Claud wondered whether the same microbes that caused intestinal inflammation could also be linked to abnormal brain development. She collected fecal samples from preterm infants and transplanted them into pregnant germ-free mice. Her initial study used growth as an indicator of overall health of the dams’ pups. When the pregnant mice received gut bacteria from infants who were struggling to gain weight, their pups grew more slowly than pups whose dams had received microbes from babies who were gaining weight normally.14 In a follow-up study, she showed that the poorly growing mice had more neuroinflammation and slower neuron development than the faster-growing mice.18
The research, Claud says, could begin to help researchers understand what differentiates a healthy microbiome in the preterm infant from an unhealthy one—and what role a child’s microbial makeup may play in driving developmental delays. “Once we understand the difference, we can try to manipulate the microbiome to move toward a more healthy state,” she says.
The Elusive Promise of Interventions
Over the past decade, researchers have used a number of experimental approaches to study gut–brain interactions in experimental animals. They have tinkered with gut microbes using treatments with antibiotics, probiotics, and fecal microbial transplants in hopes of identifying potential therapies for illnesses that may be mediated by the microbiome.
GI symptoms ranging from chronic constipation to inflammatory bowel disease are common in people with ASDs.19 The causes of these problems are unclear, but there is some evidence that altered intestinal flora may be involved. For example, in January 2017, a small trial in children diagnosed with ASDs provided preliminary evidence that changes to the gut flora may affect autism symptoms.20 The study compared 18 children with ASD diagnoses and severe gastrointestinal GI problems with a control group of 20 children who had neither ASD diagnoses nor GI problems. At baseline, the neurotypical children had much more diverse gut microbiomes than the children with ASDs.
The study team, led by researchers at Arizona State and the University of Arizona, showed that the children with ASDs scored better on assessments of both GI and autism symptoms after they received infusions of gut bacteria from healthy donors. These children’s microbiomes also became more diverse, comparable to the controls. Assessments of age-appropriate behavior at baseline and after treatment showed that the developmental age of the children increased, on average, by 1.4 years, although they still scored below their chronological ages. However, although the new study suggests the microbiome could be a therapeutic target for ASD research and treatment, the findings must first be replicated in randomized controlled trials.
Irritable bowel syndrome (IBS) is another condition with an apparent gut–brain connection. People with IBS often suffer from anxiety and depression along with GI symptoms such as abdominal pain, bloating, diarrhea, or constipation.21 Studies on the beneficial effects of manipulating the gut flora in patients with IBS have proved largely inconclusive, though some analyses suggest that certain probiotics may help some patients.22 It is also still unclear whether provocative findings in germ-free mice might eventually translate into clinical therapies. “We’re still very much in the early days of all of this,” says John Cryan, a neuroscientist and microbiome researcher at University College Cork in Ireland.
Studies in germ-free mice suggest that microbial interventions during the early postnatal period—while the microbiome is still developing—may have positive lifelong impacts on gut flora and neurological health.8,9 However, potential benefits of intervention in adulthood remain less clear.10 Once the architecture of the core microbiome is established, there may be some opportunities to manipulate the microbiome, Mayer says, but only “within a certain bandwidth of what was set up early in life.”
In a small 2013 trial of 36 healthy women, Mayer and colleagues showed that those who took a yogurt-based probiotic over four weeks had a diminished response to anxiety-producing stimuli, in comparison with women who took a placebo.23 Other small studies of probiotic interventions have shown modest associations with improved mood and variable results with respect to cognition.24
More recently, Mayer and the research team at the University of California, Los Angeles, studied fecal samples collected from 40 women. They found that women whose gut microbiomes were dominated by one set of bacteria behaved differently and had slight structural differences in a part of the brain involved in memory, in comparison with those study participants whose microbiomes were dominated by a different set of bacteria.25 However, it is unknown whether brain and behavior differences might be a cause or a result of differences in the gut microbiome—or, indeed, whether the observed associations are simply coincidental.
Many microbiome researchers now are beginning to do studies to see if animal findings are relevant to humans. Yet, some researchers caution that translational studies may be getting ahead of the basic research.26 “We know we see differences and changes in behavior and differences in brain function, but how that happens, we do not know,” says Paul Forsythe, a neuroimmunologist at McMaster University in Ontario, Canada.
A deeper understanding of how the nervous and immune systems transmit signals from the gut to the brain may help researchers parse which types of interventions are worth pursuing, says Forsythe. Clues to potential relevant pathways and mechanisms are emerging. One proposed pathway is facilitation of signaling through the vagus nerve, which extends from the abdomen to the brainstem.27 Microbes also have been shown to be the primary producer of serotonin,28 a neurotransmitter that plays a key role in neurodevelopment, transmits impulses between nerve cells, and helps maintain mood balance.29
Environmental Health through the Microbial Lens
Animal studies have shown that environmental chemicals, including triclosan,30 polychlorinated biphenyls,31 arsenic,32 and diazinon,33 can cause changes in the composition and functional capacity of the gut microbiome. These chemicals are also known or suspected neurotoxicants.34,35,36,37 “There are definitely threads suggesting a link between the microbiome and some [neurological] disorders. Environmental health researchers now are starting to tie those threads together,” says Lisa Chadwick, a program administrator in the NIEHS Division of Extramural Research and Training.
Kun Lu, a toxicologist at the University of North Carolina at Chapel Hill, looks at chemical exposures through a microbial lens. He studies what changes in gut bacteria function mean for the neurotoxicity of certain environmental chemicals.
Researchers had previously observed that organophosphates—a class of compounds that include potent nerve agents and pesticides—cause more apparent neurotoxicity in male rodents than in female rodents.38,39,40 Lu also knew there were significant differences in the structure and function of gut microbiome between males and females.41 He wondered whether changes in the microbiome played a role in the sex-specific neurotoxicity of organophosphate pesticides.

Laboratory research has shown that several environmental chemicals can change the composition and functional capacity of the gut microbiome. For example, studies in mice showed that diazinon, an organophosphate pesticide, altered the animals’ microbiomes in sex-specific ways, with males affected more negatively than females. The implications for humans are unknown. Image: © pailoolom/iStockphoto.
At sufficient doses, organophosphate pesticides, such as diazinon, curb the activity of acetylcholinesterase, an enzyme that breaks down the neurotransmitter acetylcholine.33 By inhibiting acetylcholinesterase, diazinon can send the nervous system into overdrive. During his earlier tenure at the University of Georgia, Lu et al. analyzed the effects of low-level diazinon exposures on the mouse microbiome.33 They hypothesized that, at the very low levels used in the study, the effects of the chemical on the microbiome could modulate the neurotoxicity of diazinon in a sex-specific manner.
The researchers found that exposure to diazinon did, in fact, alter the microbiomes of both male and female mice in sex-specific ways. For example, after diazinon exposure, several harmful bacteria strains were detected in the male gut, but not in the female gut. Metagenomic and metabolomic sequencing showed that differences in how diazinon altered the metabolic function of the animals’ gut bacteria—including the activity of bacterial genes involved in the synthesis and regulation of neurotransmitters—were highly sex-specific. Lu says the gut microbiome may be a player in the neurotoxic effects of other environmental chemicals, too. He has also found that chemicals such as nicotine42 and arsenic43,44 can alter the function of the microbiome in a sex-specific manner.
Matt Rand, a toxicologist at the University of Rochester Medical Center, says gut microbes also may play a role in how quickly the body eliminates methylmercury.45,46 That’s important, says Rand, because “slower or faster elimination can drastically influence how much mercury accumulates in your body if you eat a lot of fish.”

Certain microbes in the gut are thought to convert methylmercury to a less toxic form that is more readily excreted. A 2012 study showed that mice treated with antibiotics to suppress their native gut flora excreted less mercury than untreated mice. If findings like these are replicated in humans, it could have important implications for people who eat a lot of fish. Image: © nobtis/iStockphoto.
Rand’s interest was piqued when he read about a decades-old experiment showing that mice fed methylmercury and antibiotics retained higher levels of the toxic chemical in their body than mice that were fed methylmercury without antibiotics.47 He wondered whether changes in gut bacteria could impact the retention of methylmercury in the human body, too.
Some gut microbes are thought to demethylate mercury, converting it to a less toxic form that is more readily excreted.48 Rand et al. showed that people with more demethylated mercury in the stool also eliminated mercury faster from the body.49 The study suggests a role for gut bacteria in mercury metabolism but does not prove a direct link between the microbiome and how quickly mercury is cleared from the body.
A small follow-up study in a group of 37 adults backed the earlier findings in mice. Rand found that two participants who were taking antibiotics eliminated methylmercury more slowly than the rest of the small cohort.49,50 Next, he plans to investigate how the gut microbiome affects methylmercury metabolism in young children and pregnant women.
“We need to continue to expand our understanding of what influence the microbiome has on infant neurodevelopment and how that works,” says Jeannie Rodriguez, project lead for the Microbiome, Environment, and Neurodevelopmental Delay study at Emory University. Rodriguez et al. are now recruiting 500 pregnant African-American women for the study, which will focus on environmental factors related to poor developmental outcomes among black babies born preterm.51 The researchers will investigate residential exposures to toxicants such as phthalates, flame retardants, and combustion by-products including polycyclic aromatic hydrocarbons. “We’re interested in how these chemicals interact with the microbiome,” says Rodriguez. “Perhaps in the future we could manipulate the microbiome in ways that would minimize toxicant exposure.”
Chadwick calls the microbiome–gut–brain axis “an exciting new area of research in environmental health.” As environmental health scientists, she says, “I think it is important for us to look back at a lot of what we think we know about environmental health through the lens of the microbiome. It may help us clear up confusion over mode of action of certain chemicals or solve other longstanding questions in environmental health.”
Figures
References
Related
Details
Cited by
Huang J, Zhang C, Wang J, Guo Q and Zou W (2019) Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats , Brain and Behavior, 10.1002/brb3.1260, (e01260)
Recommended
Back